实用医学杂志 ›› 2024, Vol. 40 ›› Issue (23): 3419-3426.doi: 10.3969/j.issn.1006-5725.2024.23.022
• 综述 • 上一篇
肖潇1,龙方懿2,王刚1
收稿日期:2024-09-02
出版日期:2024-12-10
发布日期:2024-12-16
通讯作者:
王刚
基金资助:Xiao XIAO1,Fangyi LONG2,Gang. WANG1
Received:2024-09-02
Online:2024-12-10
Published:2024-12-16
Contact:
Gang. WANG
摘要:
细胞焦亡(pyroptosis)是一种具有炎症特征的程序性细胞死亡方式,近年来在生物医学领域引起了广泛关注。细胞焦亡在激活宿主防御、促进炎症反应、调节免疫应答及影响肿瘤微环境等方面发挥重要作用。在癌症的研究中,细胞焦亡的双重作用尤为显著,它既可以抑制肿瘤生长,也能在某些情况下促进肿瘤的发展。此外,对细胞焦亡的研究为癌症治疗提供了新的视角,特别是在增强传统放化疗效果、促进抗肿瘤免疫反应以及开发纳米治疗策略方面,细胞焦亡的机制为新研究途径提供了基础。该文综述了细胞焦亡在癌症进展中的调控作用,以及其作为潜在抗癌策略的理论基础和实际应用进展,期望为未来癌症治疗策略的发展提供新的思路和方向。
中图分类号:
肖潇,龙方懿,王刚. 细胞焦亡在癌症治疗中的双重作用与新策略[J]. 实用医学杂志, 2024, 40(23): 3419-3426.
Xiao XIAO,Fangyi LONG,Gang. WANG. Dual role and new strategies of pyroptosis in cancer therapy[J]. The Journal of Practical Medicine, 2024, 40(23): 3419-3426.
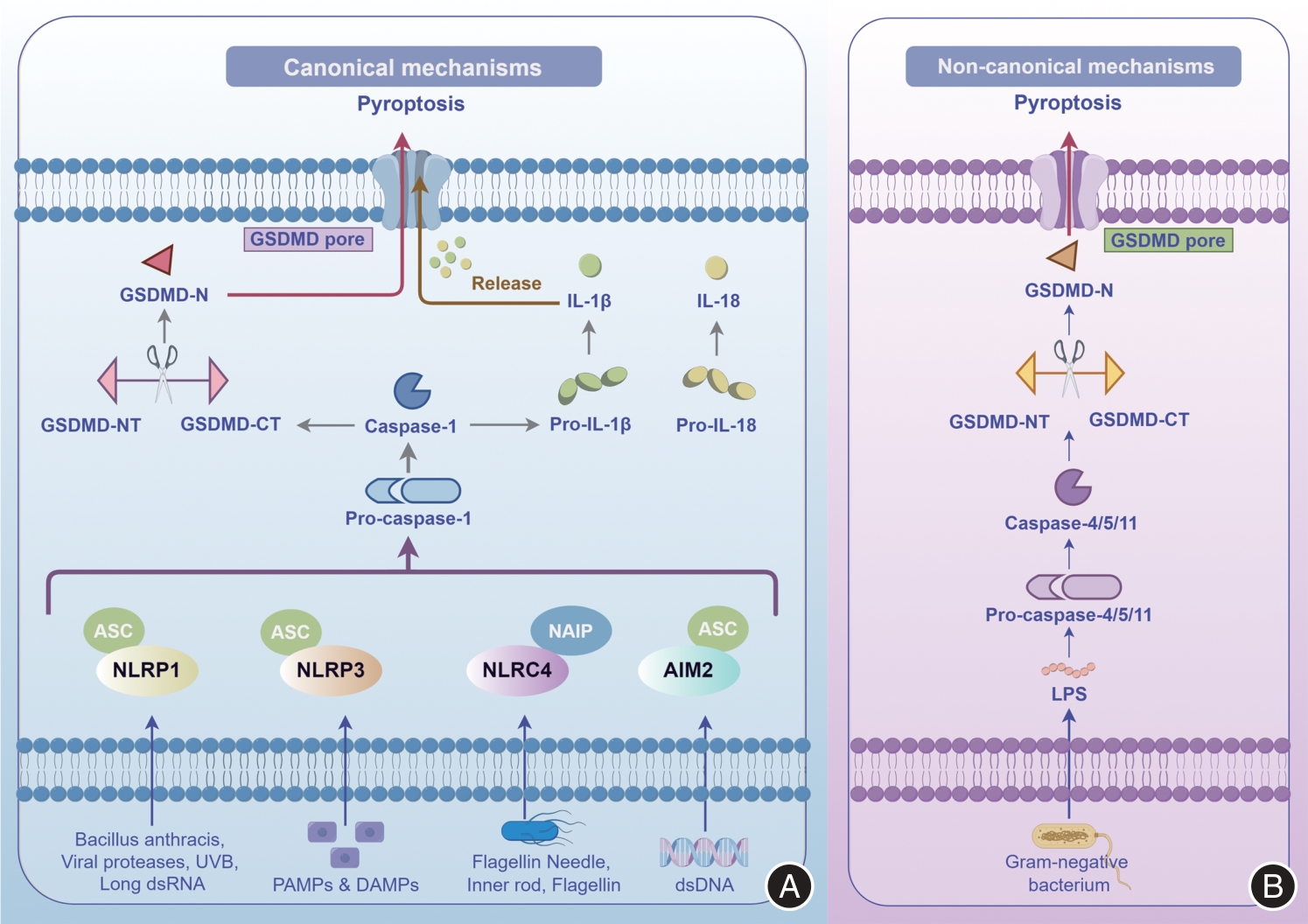
图1
细胞焦亡的两种主要途径注:A,依赖Caspase-1的经典途径。在经典途径中,病原体或内源性危险信号分别触发NLRP1、NLRP3、NLRC4、AIM2等模式识别受体(PRR),并与接头蛋白ASC和无活性的Caspase-1前体(Pro-caspase-1)组装形成炎性小体复合物。随后,活化的Caspase-1裂解GSDMD和Pro-IL1β/Pro-IL18,GSDMD的N端结构域(GSDMD-NT)在质膜中形成非选择性孔隙,并释放细胞内容物,从而执行细胞焦亡。B,依赖Caspase-4/5/11的非经典途径。细胞外革兰阴性菌的脂多糖(LPS)直接激活Pro-caspase-4/5/11,这些活化的Caspase进一步裂解GSDMD生成GSDMD-NT,从而触发细胞焦亡(本图由Figdraw绘制)"
| 1 |
SHI J, GAO W, SHAO F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death[J]. Trends Biochem Sci, 2017, 42(4):245-254. doi:10.1016/j.tibs.2016.10.004
doi: 10.1016/j.tibs.2016.10.004 |
| 2 |
VASUDEVAN S O, BEHL B, RATHINAM V A. Pyroptosis-induced inflammation and tissue damage[J]. Semin Immunol, 2023, 69:101781. doi:10.1016/j.smim.2023.101781
doi: 10.1016/j.smim.2023.101781 |
| 3 |
WEI Y, YANG L, PANDEYA A, et al. Pyroptosis-Induced Inflammation and Tissue Damage[J]. J Mol Biol, 2022, 434(4):167301. doi:10.1016/j.jmb.2021.167301
doi: 10.1016/j.jmb.2021.167301 |
| 4 |
FANG Y, TANG Y, HUANG B. Pyroptosis: A road to next-generation cancer immunotherapy[J]. Semin Immunol, 2023, 68:101782. doi:10.1016/j.smim.2023.101782
doi: 10.1016/j.smim.2023.101782 |
| 5 |
FARIA S S, COSTANTINI S, DE LIMA V, et al. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer[J]. J Biomed Sci, 2021, 28(1):26. doi:10.1186/s12929-021-00724-8
doi: 10.1186/s12929-021-00724-8 |
| 6 |
COOKSON B T, BRENNAN M A. Pro-inflammatory programmed cell death[J]. Trends Microbiol, 2001, 9(3):113-114. doi:10.1016/s0966-842x(00)01936-3
doi: 10.1016/s0966-842x(00)01936-3 |
| 7 |
GALLUZZI L, LOPEZ-SOTO A, KUMAR S, et al. Caspases Connect Cell-Death Signaling to Organismal Homeostasis[J]. Immunity, 2016,44(2):221-231. doi:10.1016/j.immuni.2016.01.020
doi: 10.1016/j.immuni.2016.01.020 |
| 8 |
XU H, YUAN Z, QIN K, et al. The molecular mechanism and evolutionary divergence of caspase 3/7-regulated gasdermin E activation[J]. Elife, 2024, 12:RP89974. doi:10.7554/elife.89974
doi: 10.7554/elife.89974 |
| 9 |
DE VASCONCELOS N M, VAN OPDENBOSCH N, VAN GORP H, et al. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture[J]. Cell Death Differ, 2019, 26(1):146-161. doi:10.1038/s41418-018-0106-7
doi: 10.1038/s41418-018-0106-7 |
| 10 |
HERR D R, YAM T, TAN W, et al. Ultrastructural Characteristics of DHA-Induced Pyroptosis[J]. Neuromolecular Med, 2020, 22(2):293-303. doi:10.1007/s12017-019-08586-y
doi: 10.1007/s12017-019-08586-y |
| 11 |
ZHANG J H, XU M. DNA fragmentation in apoptosis[J]. Cell Res, 2000, 10(3):205-211. doi:10.1038/sj.cr.7290049
doi: 10.1038/sj.cr.7290049 |
| 12 |
DEVANT P, KAGAN J C. Molecular mechanisms of gasdermin D pore-forming activity[J]. Nat Immunol, 2023, 24(7):1064-1075. doi:10.1038/s41590-023-01526-w
doi: 10.1038/s41590-023-01526-w |
| 13 |
MOLLA M D, AKALU Y, GETO Z, et al. Role of Caspase-1 in the Pathogenesis of Inflammatory-Associated Chronic Noncommunicable Diseases[J]. J Inflamm Res, 2020, 13:749-764. doi:10.2147/jir.s277457
doi: 10.2147/jir.s277457 |
| 14 |
RAO Z, ZHU Y, YANG P, et al. Pyroptosis in inflammatory diseases and cancer[J]. Theranostics, 2022, 12(9):4310-4329. doi:10.7150/thno.71086
doi: 10.7150/thno.71086 |
| 15 |
LIU Y R, WANG J Q, LI J. Role of NLRP3 in the pathogenesis and treatment of gout arthritis[J]. Front Immunol, 2023, 14:1137822. doi:10.3389/fimmu.2023.1137822
doi: 10.3389/fimmu.2023.1137822 |
| 16 |
LI C, CHEN M, HE X, et al. A mini-review on ion fluxes that regulate NLRP3 inflammasome activation[J]. Acta Biochim Biophys Sin (Shanghai), 2021, 53(2):131-139. doi:10.1093/abbs/gmaa155
doi: 10.1093/abbs/gmaa155 |
| 17 |
CHAVARRIA-SMITH J, VANCE R E. The NLRP1 inflammasomes[J]. Immunol Rev, 2015,265(1):22-34. doi:10.1111/imr.12283
doi: 10.1111/imr.12283 |
| 18 |
DEVI S, STEHLIK C, DORFLEUTNER A. An Update on CARD Only Proteins (COPs) and PYD Only Proteins (POPs) as Inflammasome Regulators[J]. Int J Mol Sci, 2020, 21(18):6901. doi:10.3390/ijms21186901
doi: 10.3390/ijms21186901 |
| 19 |
LU A, WU H. Structural mechanisms of inflammasome assembly[J]. FEBS J, 2015, 282(3):435-444. doi:10.1111/febs.13133
doi: 10.1111/febs.13133 |
| 20 |
BARNETT K C, LI S, LIANG K, et al. A 360 degrees view of the inflammasome: Mechanisms of activation, cell death, and diseases[J]. Cell, 2023, 186(11):2288-2312. doi:10.1016/j.cell.2023.04.025
doi: 10.1016/j.cell.2023.04.025 |
| 21 |
MATIKAINEN S, NYMAN T A, CYPRYK W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome[J]. J Immunol, 2020, 204(12):3063-3069. doi:10.4049/jimmunol.2000373
doi: 10.4049/jimmunol.2000373 |
| 22 |
WANG D, ZHANG B, LIU X, et al. Agree to disagree: The contradiction between IL-18 and IL-37 reveals shared targets in cancer[J]. Pharmacol Res, 2024, 200:107072. doi:10.1016/j.phrs.2024.107072
doi: 10.1016/j.phrs.2024.107072 |
| 23 |
MOHAMMED T F, QADIR F A. Detection of IL-1beta, VEGF and IL-4 with their novel genetic variations in breast cancer patients[J]. Saudi J Biol Sci, 2023, 30(2):103544. doi:10.1016/j.sjbs.2022.103544
doi: 10.1016/j.sjbs.2022.103544 |
| 24 |
WANG F, LI G, NING J, et al. Alcohol accumulation promotes esophagitis via pyroptosis activation[J]. Int J Biol Sci, 2018,14(10):1245-1255. doi:10.7150/ijbs.24347
doi: 10.7150/ijbs.24347 |
| 25 | GAO J, QIU X, XI G, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non‑small cell lung cancer[J]. Oncol Rep, 2018,40(4):1971-1984. |
| 26 |
LI S, YUE M, XU H, et al. Chemotherapeutic drugs-induced pyroptosis mediated by gasdermin E promotes the progression and chemoresistance of pancreatic cancer[J]. Cancer Lett, 2023, 564:216206. doi:10.1016/j.canlet.2023.216206
doi: 10.1016/j.canlet.2023.216206 |
| 27 | YU S, YIN J J, MIAO J X, et al. Activation of NLRP3 inflammasome promotes the proliferation and migration of esophageal squamous cell carcinoma[J]. Oncol Rep, 2020, 43(4):1113-1124. |
| 28 |
JIANG X, ZHU Z, DING L, et al. ALKBH4 impedes 5-FU Sensitivity through suppressing GSDME induced pyroptosis in gastric cancer[J]. Cell Death Dis, 2024,15(6):435. doi:10.1038/s41419-024-06832-1
doi: 10.1038/s41419-024-06832-1 |
| 29 |
GUO J, YE F, XIE W, et al. The HOXC-AS2/miR-876-5p/HKDC1 axis regulates endometrial cancer progression in a high glucose-related tumor microenvironment[J]. Cancer Sci, 2022, 113(7):2297-2310. doi:10.1111/cas.15384
doi: 10.1111/cas.15384 |
| 30 |
LIU Y, FANG Y, CHEN X, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome[J]. Sci Immunol, 2020, 5(43):eaax7969. doi:10.1126/sciimmunol.aax7969
doi: 10.1126/sciimmunol.aax7969 |
| 31 |
DING Y, YAN Y, DONG Y, et al. NLRP3 promotes immune escape by regulating immune checkpoints: A pan-cancer analysis[J]. Int Immunopharmacol, 2022, 104:108512. doi:10.1016/j.intimp.2021.108512
doi: 10.1016/j.intimp.2021.108512 |
| 32 |
DENG M, SUN S, ZHAO R, et al. The pyroptosis-related gene signature predicts prognosis and indicates immune activity in hepatocellular carcinoma[J]. Mol Med, 2022, 28(1):16. doi:10.1186/s10020-022-00445-0
doi: 10.1186/s10020-022-00445-0 |
| 33 |
HU Y, LIU Y, ZONG L, et al. The multifaceted roles of GSDME-mediated pyroptosis in cancer: Therapeutic strategies and persisting obstacles[J]. Cell Death Dis, 2023, 14(12):836. doi:10.1038/s41419-023-06382-y
doi: 10.1038/s41419-023-06382-y |
| 34 |
XIA J, CHU C, LI W, et al. Mitochondrial Protein UCP1 Inhibits the Malignant Behaviors of Triple-negative Breast Cancer through Activation of Mitophagy and Pyroptosis[J]. Int J Biol Sci, 2022, 18(7):2949-2961. doi:10.7150/ijbs.68438
doi: 10.7150/ijbs.68438 |
| 35 |
YANG C, WANG Z Q, ZHANG Z C, et al. CBL0137 activates ROS/BAX signaling to promote caspase-3/GSDME-dependent pyroptosis in ovarian cancer cells[J]. Biomed Pharmacother, 2023, 161:114529. doi:10.1016/j.biopha.2023.114529
doi: 10.1016/j.biopha.2023.114529 |
| 36 |
YU D, WANG S, WANG J, et al. EZH2-STAT3 signaling pathway regulates GSDMD-mediated pyroptosis in glioblastoma[J]. Cell Death Discov, 2024, 10(1):341. doi:10.1038/s41420-024-02105-0
doi: 10.1038/s41420-024-02105-0 |
| 37 |
TENG J F, MEI Q B, ZHOU X G, et al. Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-kappaB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer[J]. Cancers (Basel), 2020, 12(1):193. doi:10.3390/cancers12010193
doi: 10.3390/cancers12010193 |
| 38 |
CAI J, YI M, TAN Y, et al. Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-IotaIota[J]. J Exp Clin Cancer Res, 2021, 40(1):190. doi:10.1186/s13046-021-01995-7
doi: 10.1186/s13046-021-01995-7 |
| 39 |
YAN H, LUO B, WU X, et al. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer[J]. Int J Biol Sci, 2021, 17(10):2606-2621. doi:10.7150/ijbs.60292
doi: 10.7150/ijbs.60292 |
| 40 |
ZHANG Z, ZHANG H, LI D, et al. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway[J]. J Cell Mol Med, 2021, 25(17):8159-8168. doi:10.1111/jcmm.16574
doi: 10.1111/jcmm.16574 |
| 41 |
WANG Y, YIN B, LI D, et al. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer[J]. Biochem Biophys Res Commun, 2018, 495(1):1418-1425. doi:10.1016/j.bbrc.2017.11.156
doi: 10.1016/j.bbrc.2017.11.156 |
| 42 |
YU J, LI S, QI J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells[J]. Cell Death Dis, 2019, 10(3):193. doi:10.1038/s41419-019-1441-4
doi: 10.1038/s41419-019-1441-4 |
| 43 |
HAGE C, HOVES S, STRAUSS L, et al. Sorafenib Induces Pyroptosis in Macrophages and Triggers Natural Killer Cell-Mediated Cytotoxicity Against Hepatocellular Carcinoma[J]. Hepatology, 2019,70(4):1280-1297. doi:10.1002/hep.30666
doi: 10.1002/hep.30666 |
| 44 |
HAN Z, LIANG Y, LI Y, et al. Programmed Cascade Polydopamine Nanoclusters for Pyroptosis-Based Tumor Immunotherapy[J]. Small, 2024, 20(42):e2401397. doi:10.1002/smll.202401397
doi: 10.1002/smll.202401397 |
| 45 |
REN Y, FENG M, HAO X, et al. USP48 Stabilizes Gasdermin E to Promote Pyroptosis in Cancer[J]. Cancer Res, 2023, 83(7):1074-1093. doi:10.1158/0008-5472.can-22-1812
doi: 10.1158/0008-5472.can-22-1812 |
| 46 |
ERKES D A, CAI W, SANCHEZ I M, et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis[J]. Cancer Discov, 2020, 10(2):254-269. doi:10.1158/2159-8290.cd-19-0672
doi: 10.1158/2159-8290.cd-19-0672 |
| 47 |
JIANG Y, YANG Y, HU Y, et al. Gasdermin D restricts anti-tumor immunity during PD-L1 checkpoint blockade[J]. Cell Rep, 2022, 41(4):111553. doi:10.1016/j.celrep.2022.111553
doi: 10.1016/j.celrep.2022.111553 |
| 48 | SANG R, FAN R, DENG A, et al. Degradation of Hexokinase 2 Blocks Glycolysis and Induces GSDME-Dependent Pyroptosis to Amplify Immunogenic Cell Death for Breast Cancer Therapy[J]. J Med Chem, 2023, 66(13):8464-8483. |
| 49 |
FONTANA P, DU G, ZHANG Y, et al. Small-molecule GSDMD agonism in tumors stimulates antitumor immunity without toxicity[J]. Cell, 2024, 187(22):6165-6181.e22. doi:10.1016/j.cell.2024.08.007
doi: 10.1016/j.cell.2024.08.007 |
| 50 |
AI Y L, WANG W J, LIU F J, et al. Mannose antagonizes GSDME-mediated pyroptosis through AMPK activated by metabolite GlcNAc-6P[J]. Cell Res, 2023, 33(12):904-922. doi:10.1038/s41422-023-00848-6
doi: 10.1038/s41422-023-00848-6 |
| 51 |
DI M, MIAO J, PAN Q, et al. OTUD4-mediated GSDME deubiquitination enhances radiosensitivity in nasopharyngeal carcinoma by inducing pyroptosis[J]. J Exp Clin Cancer Res, 2022, 41(1):328. doi:10.1186/s13046-022-02533-9
doi: 10.1186/s13046-022-02533-9 |
| 52 |
SU L, CHEN Y, HUANG C, et al. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models[J]. Sci Transl Med, 2023, 15(678):eabl7895. doi:10.1126/scitranslmed.abl7895
doi: 10.1126/scitranslmed.abl7895 |
| 53 |
LI Y T, TAN X Y, MA L X, et al. Targeting LGSN restores sensitivity to chemotherapy in gastric cancer stem cells by triggering pyroptosis[J]. Cell Death Dis, 2023, 14(8):545. doi:10.1038/s41419-023-06081-8
doi: 10.1038/s41419-023-06081-8 |
| 54 | 韩蓉,吴东明,邓任华,等. X线辐照激活NLRP3灰性体引起损伤肺组织细胞焦亡(pyroptosis)[J]. 细胞与分子免疫学杂志, 2017, 33(9):1206-1211. |
| 55 |
YANG C, SONG C, WANG Y, et al. Re-Du-Ning injection ameliorates radiation-induced pneumonitis and fibrosis by inhibiting AIM2 inflammasome and epithelial-mesenchymal transition[J]. Phytomedicine, 2022, 102:154184. doi:10.1016/j.phymed.2022.154184
doi: 10.1016/j.phymed.2022.154184 |
| 56 |
QIU H, WANG W, HU K, et al. EuHD1 protects against inflammatory injury driven by NLRP3 inflammasome[J]. Int Immunopharmacol, 2023, 115:109712. doi:10.1016/j.intimp.2023.109712
doi: 10.1016/j.intimp.2023.109712 |
| 57 |
ZHANG L, BAI H, ZHOU J, et al. Role of tumor cell pyroptosis in anti-tumor immunotherapy[J]. Cell Insight, 2024, 3(3):100153. doi:10.1016/j.cellin.2024.100153
doi: 10.1016/j.cellin.2024.100153 |
| 58 |
LI M, JIANG P, YANG Y, et al. The role of pyroptosis and gasdermin family in tumor progression and immune microenvironment[J]. Exp Hematol Oncol, 2023, 12(1):103. doi:10.1186/s40164-023-00464-5
doi: 10.1186/s40164-023-00464-5 |
| 59 |
NING H, HUANG S, LEI Y, et al. Enhancer decommissioning by MLL4 ablation elicits dsRNA-interferon signaling and GSDMD-mediated pyroptosis to potentiate anti-tumor immunity[J]. Nat Commun, 2022, 13(1):6578. doi:10.1038/s41467-022-34253-1
doi: 10.1038/s41467-022-34253-1 |
| 60 |
WU F, WANG M, ZHONG T, et al. Inhibition of CDC20 potentiates anti-tumor immunity through facilitating GSDME-mediated pyroptosis in prostate cancer[J]. Exp Hematol Oncol, 2023, 12(1):67. doi:10.1186/s40164-023-00428-9
doi: 10.1186/s40164-023-00428-9 |
| 61 |
WU L, BAI S, HUANG J, et al. Nigericin Boosts Anti-Tumor Immune Response via Inducing Pyroptosis in Triple-Negative Breast Cancer[J]. Cancers (Basel), 2023, 15(12):3221. doi:10.3390/cancers15123221
doi: 10.3390/cancers15123221 |
| 62 |
ZHANG Z, ZHANG Y, XIA S, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity[J]. Nature, 2020, 579(7799):415-420. doi:10.1038/s41586-020-2071-9
doi: 10.1038/s41586-020-2071-9 |
| 63 |
SONG X, HUANG H, XIA L, et al. Engineering 2D Multienzyme-Mimicking Pyroptosis Inducers for Ultrasound-Augmented Catalytic Tumor Nanotherapy[J]. Adv Sci (Weinh), 2023, 10(24):e2301279. doi:10.1002/advs.202301279
doi: 10.1002/advs.202301279 |
| 64 |
CHANG M, WANG Z, DONG C, et al. Ultrasound-Amplified Enzyodynamic Tumor Therapy by Perovskite Nanoenzyme-Enabled Cell Pyroptosis and Cascade Catalysis[J]. Adv Mater, 2023, 35(7):e2208817. doi:10.1002/adma.202208817
doi: 10.1002/adma.202208817 |
| 65 |
XU K, CHANG M, WANG Z, et al. Multienzyme-Mimicking LaCoO(3) Nanotrigger for Programming Cancer-Cell Pyroptosis[J]. Adv Mater, 2023, 35(35):e2302961. doi:10.1002/adma.202302961
doi: 10.1002/adma.202302961 |
| 66 |
LI F, ZHANG X Q, HO W, et al. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy[J]. Nat Commun, 2023, 14(1):4223. doi:10.1038/s41467-023-39938-9
doi: 10.1038/s41467-023-39938-9 |
| 67 |
CHEN B, YAN Y, YANG Y, et al. A pyroptosis nanotuner for cancer therapy[J]. Nat Nanotechnol, 2022, 17(7):788-798. doi:10.1038/s41565-022-01125-0
doi: 10.1038/s41565-022-01125-0 |
| 68 |
DING B, CHEN H, TAN J, et al. ZIF-8 Nanoparticles Evoke Pyroptosis for High-Efficiency Cancer Immunotherapy[J]. Angew Chem Int Ed Engl, 2023, 62(10):e202215307. doi:10.1002/anie.202215307
doi: 10.1002/anie.202215307 |
| 69 |
WANG M, WU M, LIU X, et al. Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer[J]. Adv Sci (Weinh), 2022, 9(29):e2202914. doi:10.1002/advs.202202914
doi: 10.1002/advs.202202914 |
| [1] | 武周游,李婷,张腾伟,房巧燕,杨刘,黎巧. 羟基壬烯醛通过抑制内皮细胞焦亡减轻新生儿脓毒症诱导的急性肺损伤[J]. 实用医学杂志, 2024, 40(2): 195-201. |
| [2] | 史秀丽,陈嘉琪,朱璠,曾娟,吴娜. miR-143-3p靶向调控TLR2/ NF-κB/NLRP3通路对溃疡性结肠炎细胞焦亡的影响[J]. 实用医学杂志, 2024, 40(15): 2056-2062. |
| [3] | 王迪,杨剑,何祥. 右美托咪定通过调控AKAP150减轻异丙酚诱导的发育期大鼠学习记忆障碍[J]. 实用医学杂志, 2024, 40(12): 1619-1624. |
| [4] | 谢丹,欧阳石. 茵陈蒿汤协同脐带间充质干细胞所释放的外泌体对急性肝衰竭及肝细胞焦亡的影响[J]. 实用医学杂志, 2023, 39(23): 3034-3042. |
| [5] | 李加善,杨德兵,彭志锋. 慢性高脂肪饮食对缺血/再灌注大鼠脑损伤影响及机制[J]. 实用医学杂志, 2023, 39(20): 2579-2583. |
| [6] | 易莎,胡楠,李粤,杨林,张玉婷,熊霞,钟桂书,陈燕. 过氧化物酶体增殖物激活受体-γ对P. acnes诱导的人角质形成细胞焦亡、增殖及凋亡的影响[J]. 实用医学杂志, 2023, 39(16): 2043-2049. |
| [7] | 刘瑾 张洁 冯莹 . 苦杏仁苷减少冠状动脉内皮细胞焦亡并改善 载脂蛋白E缺陷小鼠动脉粥样硬化斑块形成 [J]. 实用医学杂志, 2023, 39(14): 1746-1755. |
| [8] | 汪乐新, 刘超, 马天龙, 杨安宁, 熊建团, 吴凯, 焦运 白志刚, 姜怡邓, 马胜超, 张旭 卢冠军, . LncRNA H19调控肾小球足细胞焦亡中作用 [J]. 实用医学杂志, 2023, 39(1): 12-20. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

